Statistics and Data Science for Pharmaceuticals
Courses and Learning Track
Training courses and learning track for data driven Pharmaceuticals looking for Biostatistics, process quality improvement and quality by design
Statistical Tools for Pharmaceuticals
This top selling popular course, covers all the statistical topics useful and required for Pharmaceuticals. The course is based on TIBCO Statistica, as it is fully compliant with every pharmaceutical guidelines. You will learn to develop sound statistical approaches to data analysis by understanding how to select the right tool for a given scenario and to correctly interpret the results of the analysis.
You will understand how to apply DOE for product/process development and transfer.
You will focus on the statistical foundations and practical applications relevant to:
-
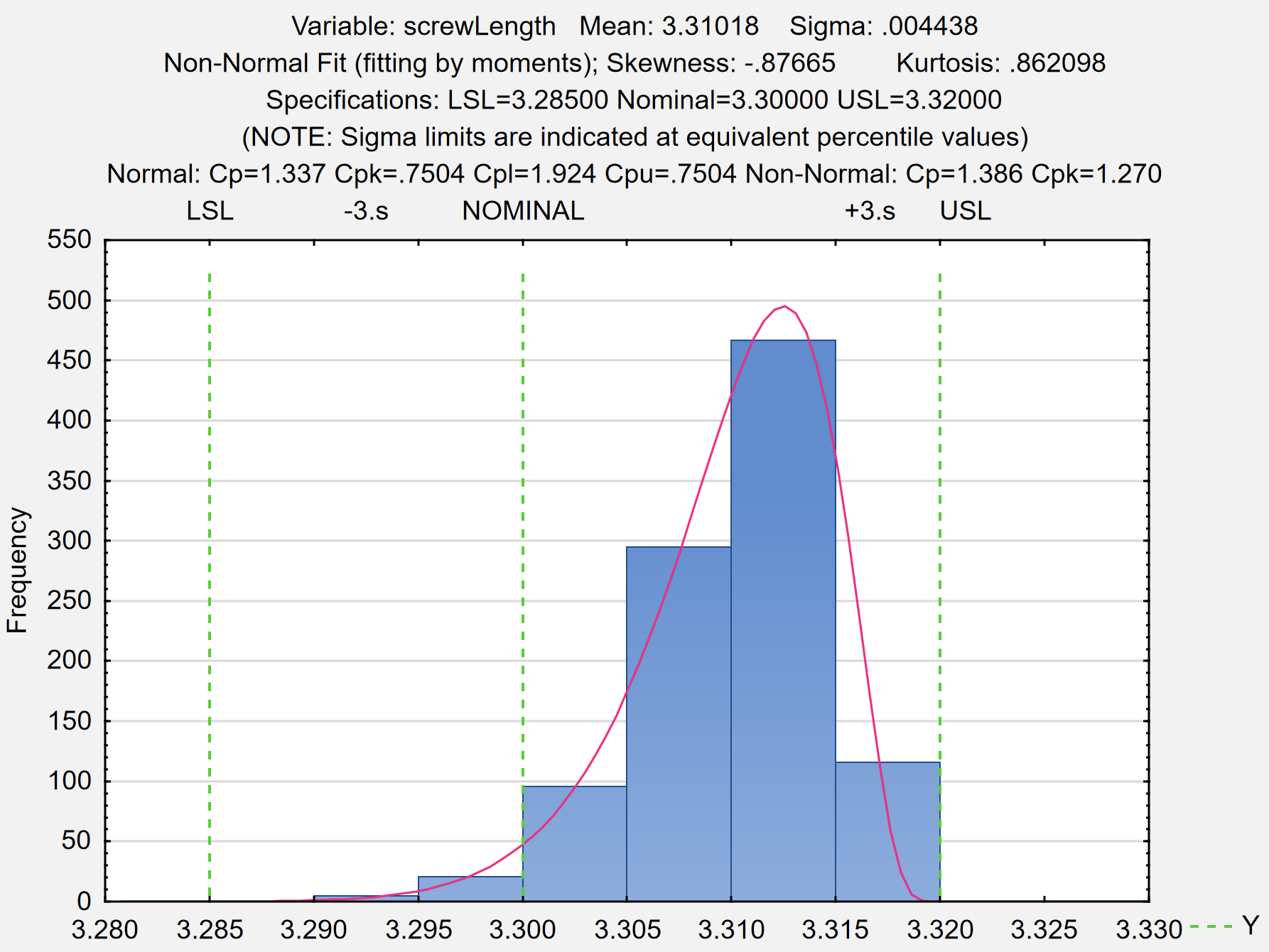
- The assessment of the Process Capability of your manufacturing process, with reference to the Critical Process Parameters (CPPs), and demonstrating that your process is maintained between the boundaries of the Design Space as studied and, therefore, does not significantly affect the Critical Quality Attributes (CQAs).
- The building of a robust statistical output for your Product Quality Reviews (PQRs)
- The evaluation of a random batch sample to determine the compliance with the specifications and whether to release, quarantine or reject it.
- The determination of DS/DP shelf life through stability data analysis
All applications place emphasis on making good business decisions based upon the practical application of statistical techniques commonly used in the pharmaceutical industry.
Topics include:
-
- Introduction to Statistics: why you need it and why you will love it by the end of the course
- Descriptive Statistics: location and variation indexes and definition of quality
- The correct graphs and the correct interpretation: box plot, histogram, bar chart, scatterplot, contour plot, probability plot
- Statistical tests and modelling: t-tests, normality test, ANOVA, regressions, equivalence tests, proportion tests, stability studies
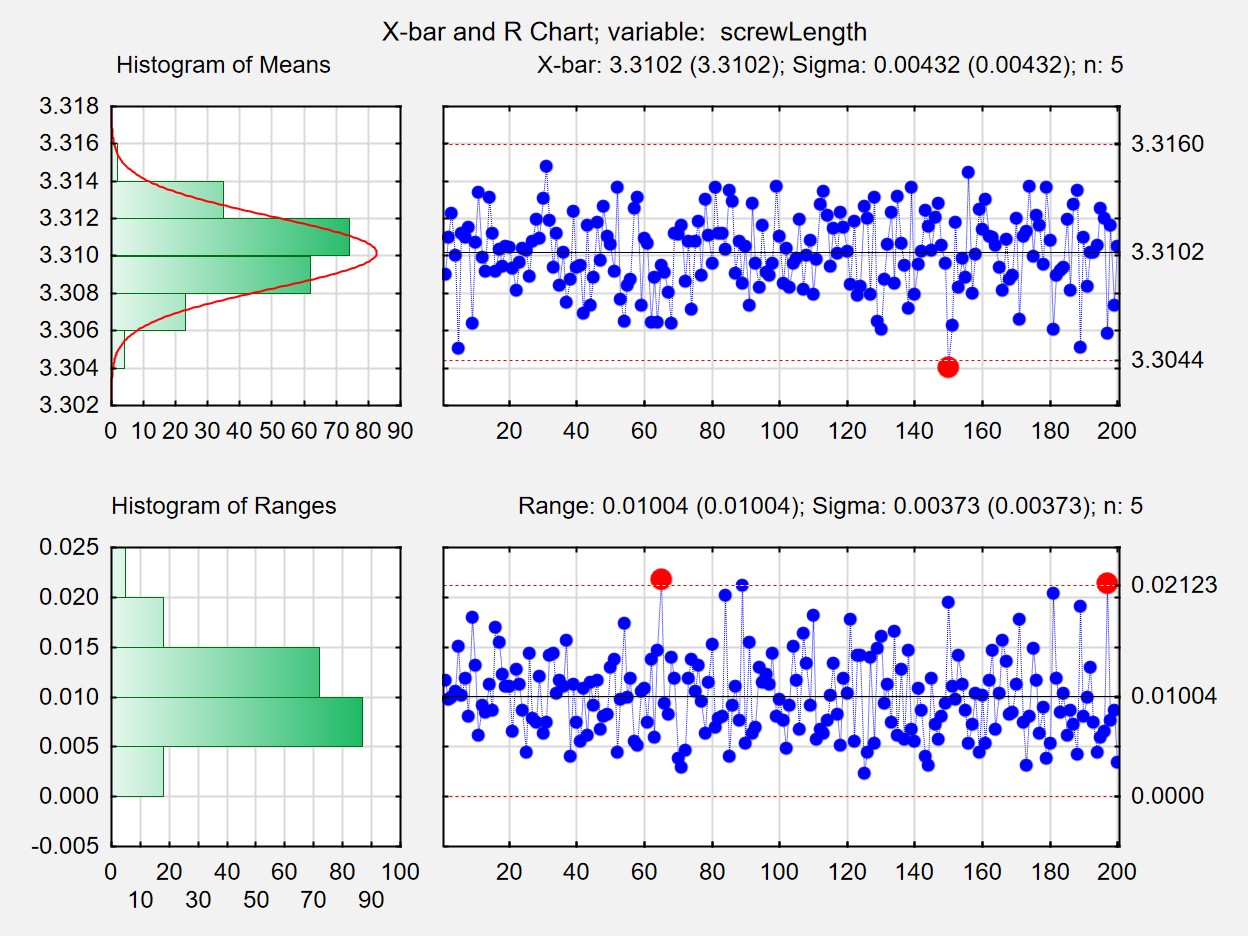
- Quality Statistics: measurement systems analysis, control charts, capability analysis, sampling plans
- Design of experiment: factorial design, sequential experimentation, response surface design
1 to 4 days, depending on desired topics.
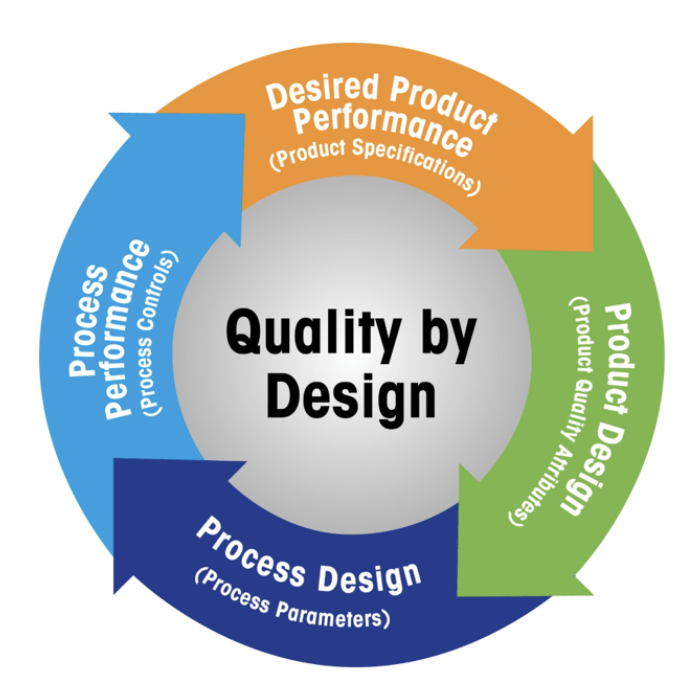

Quality by Design
The FDA imperative is outlined in its report “Pharmaceutical Quality for the 21st Century: A Risk-Based Approach.” In the past few years, the agency has implemented the concepts of QbD into its pre-market processes. The goal of QbD is to built the quality into the final product (DS or DP), starting from the development stage and so that the product itself results to be safe, efficacious and affordable to the patient. The full understanding of the product and process by which it is manufactured, along with the associated risk management strategy, forms the roadmap to hit the aforementioned goal.
This two days course will guide you through a full and practical understanding of QbD concepts and guidelines.
Topics include:
DAY 1
-
- QbD approach: Background, history and drivers
- Benefits of the QbD approach
- Regulatory frame: ICH guidelines series
- Steps to define QTPP, CQAs AND CPPs
- Interactive session: QTPP, CQAs AND CPPs typical points of discussion within a team
DAY 2
-
- Product & process design: must have” vs “nice-to-have”
- Design space and control strategy definition
- QbD approach for technology transfer and scale up
- QbD approach for process validation
- Case studies: QbD in practice
Duration: 2 days.
Before this course, you may want some training in Basic Statistics and Design of Experiment.

Data Integrity
Data integrity is the maintenance of, and the assurance of the accuracy and consistency of data over its entire life-cycle and is a critical aspect to the design, implementation and usage of any system which stores, processes, or retrieves data. The U.S. Food and Drug Administration has created draft guidance on data integrity for the pharmaceutical manufacturers required to adhere to U.S. Code of Federal Regulations 21 CFR Parts 210–212. This training will guide you through the essentials of data culture and give every details about guidelines, tools and best practices in order to grant integrity to your data.
Topics include:
-
- Data Integrity and Good Documentation
- Data Integrity
- Laboratory Data Integrity: Meeting FDA & EU Concerns
- Data Integrity Master Class
- Audit Trail Review
- Audit Trail Review for Comp. Systems in Analytical Laboratories
- Raw Data: Understanding, Defining and Managing
- Data Integrity Quality Oversight in the GMP Regulated QC Laboratory AND Audit Trail Review Workshop
- Lab Data Integrity Master Class
- D.I.C.T. – Data Integrity in Clinical Trials
Duration: 2 days.
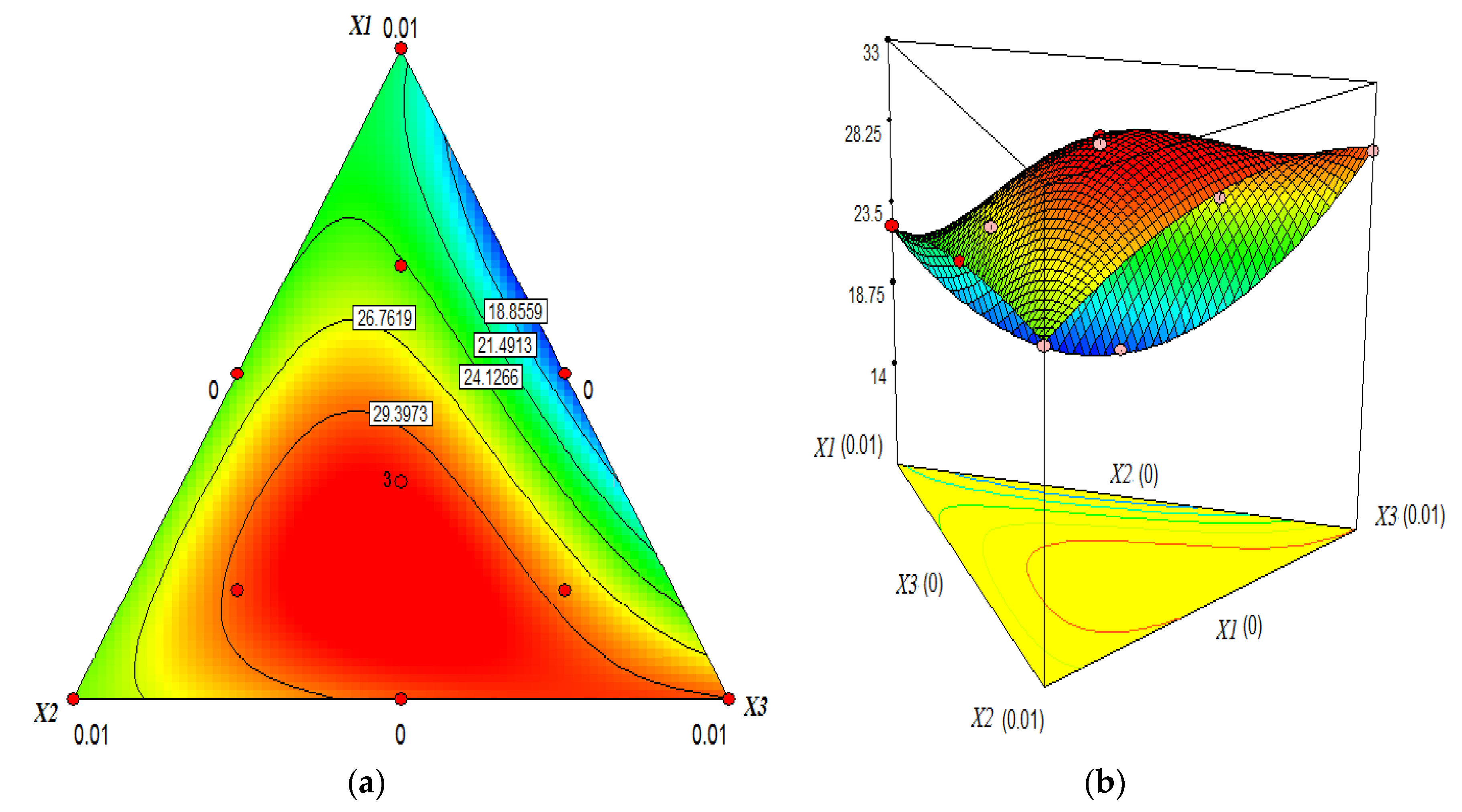
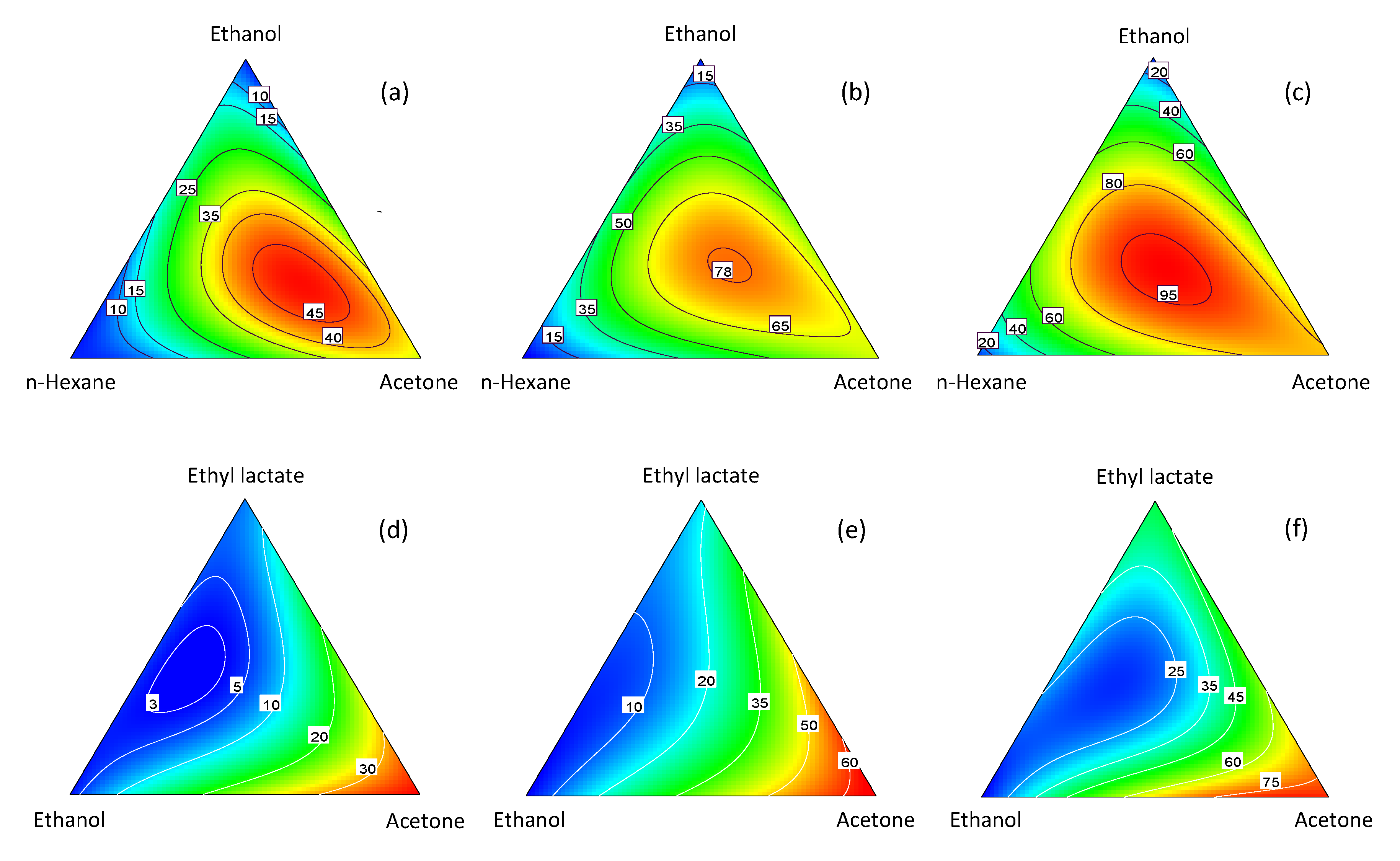
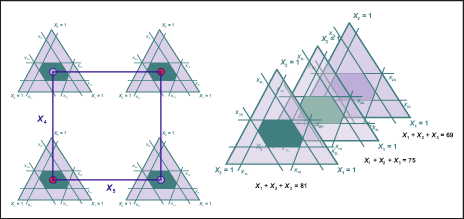
Formulation Designs
Which chemical formulation optimises a given response? Statistical learning and design of experiment are the best, safest and fastest way to find the answer.
Formulation Design is an advanced type of DOE that investigates the effect of ingredient proportions on one or more responses. This approach will let you find the best formulations that optimise your product. The course will give you all the details you need to design, run and analyse a Formulation Design experiment. Also, you will learn how to go even further with your model, predicting the process capability relative to every candidate best formulation.
Topics include:
-
- Introduction to Formulation Design
- Simplex Lattice and Centroid Designs
- Upper and Lower Constraints
- Pseudocomponents
- Extreme Vertices Designs
- Mixture-Process Variable designs
- Mixture-Amount Designs
Duration: 2 days.
Before this course, you may want some training in Basic Statistics and Design of Experiment.
Onsite or online
Courses can be either onsite or online, we are prepared for both cases!
Custom programs
We can adapt the programs to meet your real needs
Italian or English
Courses can be either in Italian or in English, in Italy or outside Italy
Training on the job
Yes, we can use also your data as examples during our courses!


Why training with us?
Our trainers actually are senior Scientists and senior Data Scientists. We do what we teach for more than 10 years, not just teach. That’s an incredibly important difference!
We have years of experience in successfully solving real use cases. And all this experience is offered to you during our training courses, helping to keep things real and practical, easy to understand and ready to be used in your business.
Luigi Roggia
A senior consultant in Data Science and one of the main experts in applied Statistics, who is holding statistical training courses since 2007. Master’s Degree in Physics, specialisation in Biophysics, European Master in Bioinformatics.
Daniela Ceriani
A senior scientist, with a distinctive career and experience in Pharmaceuticals. Master’s Degree in Chemistry and Pharmaceutical Technologies, specialised in Quality by Design, Technology Transfer and Data Integrity.
